Sooma Medical Awarded US Patent for Advanced Stimulation Electrode
11.01.2019
Official Press Release
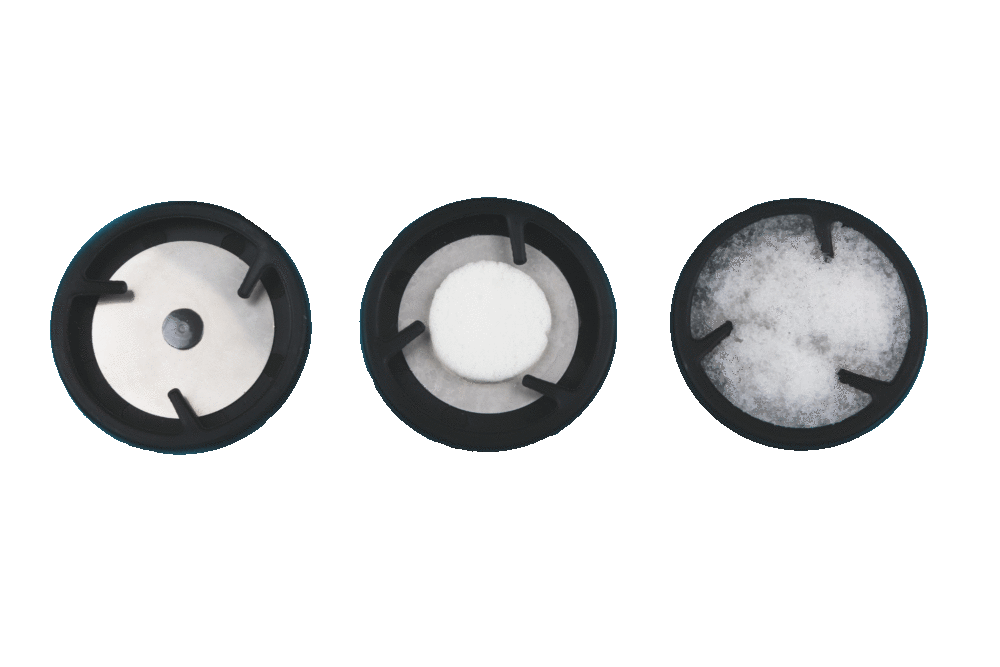
Helsinki, Finland, January 11, 2019 (Newswire.com) – Sooma Medical announces receipt of a U.S. patent for it’s new stimulation electrode.The electrode technology is essential for safe and effective delivery of all neuromodulation therapies.The electrode technology developed by Sooma R&D team dramatically reduces the resistance in the skin-electrode interface when compared to competing solutions. Further benefits of the patented technology are faster patient preparation and superior accuracy in delivery of the stimulation dose. The hydrophilic properties of the material ensure stable contact for extended periods of time.
This technology is the result of a long term research project with industrial and academic partners finally reaching product stage. The company strategy is to keep the focus of technological innovations where it matters most to the patient and the clinical user: ease of use and patient safety. Since the launch of the new electrode more than 1000 patients worldwide have received Sooma tDCS treatment in more than 20.000 treatment sessions.
With yet another patent secured in the IP portfolio, Sooma Medical strengthens it’s position as the leading provider of home-based neuromodulation solutions with a unique product portfolio.
Sooma tDCS is a therapy product using weak electrical stimulation (transcranial direct currentstimulation, tDCS) and is intended for treatment of major depressive disorder (MDD), chronic pain and fibromyalgia. The devices are used by professionals and patients in over 100 clinics worldwide. In a recently published report, 59% of patients suffering from major depression obtained a treatment response after only 3 weeks of treatment. Sooma Medical product portfolio also includes a digital platform for remote supervision of patient compliance, behavioral changes and treatment outcome.
Sooma Medical has obtained regulatory approvals for the product in the following markets: EU (Class IIa), Canada, Mexico, Singapore, Australia, Malaysia, Indonesia and Turkey. In the U.S. the FDA limits the use of the tDCS for investigational use only. Sooma products are distributed in over 30 countries.
Video about the use of the new electrode here.
CONTACTS: Tuomas Neuvonen, CEO
info@soomamedical.com
tel. +358 10 328 9811
REF: U.S. patent 10,173,049
Original Source: www.newswire.com
Latest news

Sooma Announces Medical Technology Expert Andreas Hartlep as New CEO
Read more
TGA approves Sooma’s at-home brain stimulation for depression in Australia
Read more
Sooma Medical Announces Pivotal FDA IDE Clinical Trial for At-Home Brain Stimulation Device for Depression Treatment
Read more