Sooma’s latest study provides the largest dataset to date on the effects of tDCS for treating MDD in real-world clinical practice

Since the publication of our 2019 white paper, we have been working on updating our clinical data regarding the effects of Sooma Therapy in the treatment of Major Depressive Disorder (MDD).
The results of such endeavour have been published as a peer-reviewed study in the Journal of Affective Disorders Reports, which contains the largest real-world dataset hitherto reported on the efficiency and tolerability of tDCS in the treatment of MDD in real-world clinical practice. You can find the study in the April issue of the Journal under the title “Reduction Of Symptoms In Patients With Major Depressive Disorder After Transcranial Direct Current Stimulation Treatment: A Real-World Study“.
This real-world study shows good tolerability and reduced depressive symptoms in patients with MDD after tDCS treatment with Sooma devices.
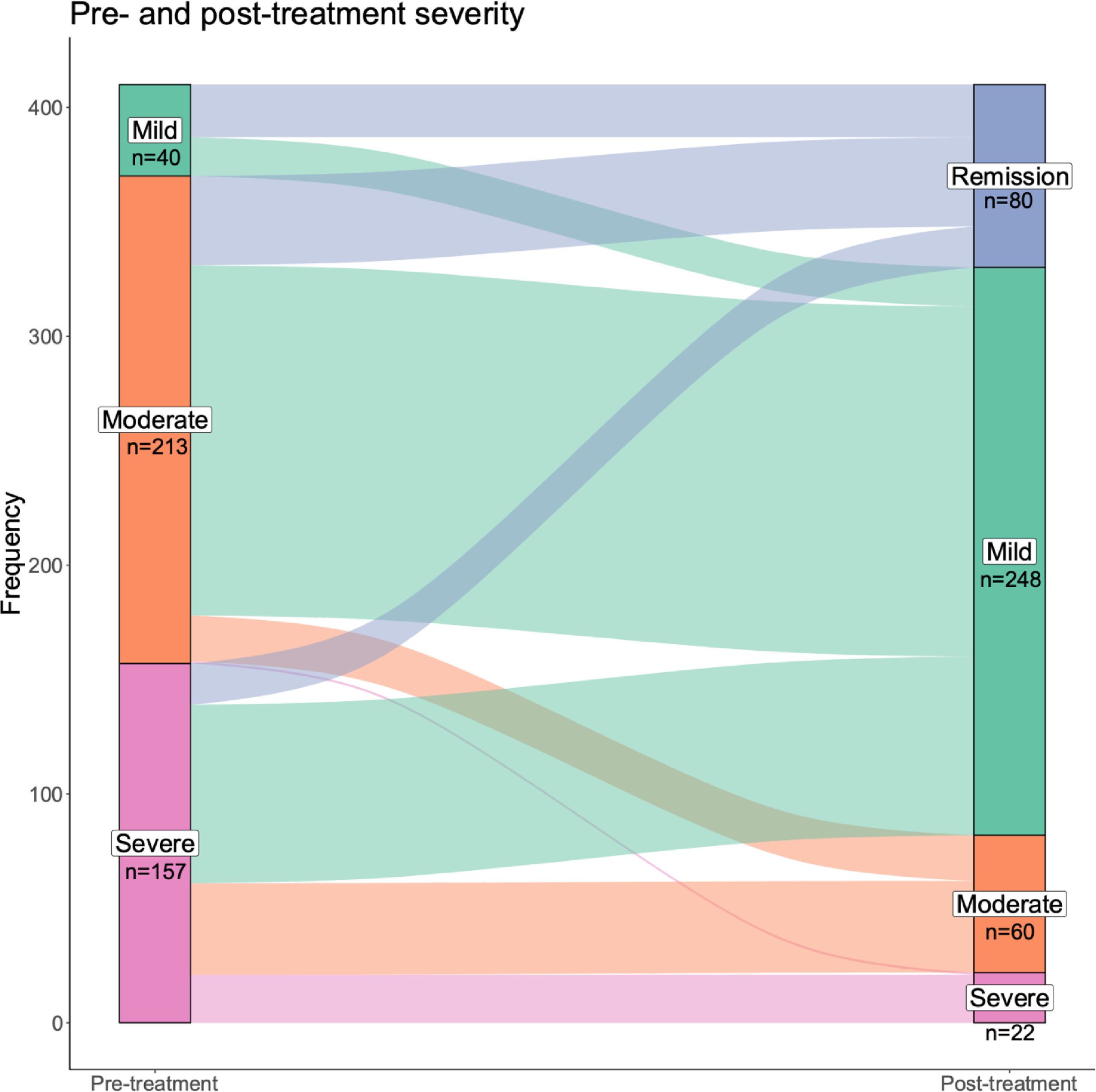
A total of 462 patients treated with Sooma tDCS as a part of routine clinical practice were enrolled in the study. A total of 410 of those patients completed the treatment course successfully. The patient’s depressive symptoms were evaluated before and after the treatment using validated depression scales (HDRS, DBI-21, MADRS, MDI).
After completing the treatment, close to 97% of all patients showed an improvement in their depression score. 55% of the patients achieved complete clinical response (CCR), remission was achieved by about 20%, and minimal clinically important difference (MCID) by close to 95%. At least half of the patients achieved CCR in all severity classes and in patients with and without concomitant use of psychotropics. However, it must be noted that the use of psychotropics or more severe depression is negatively associated with outcomes.
No serious adverse effects were reported during the treatment. The most common adverse effects observed were skin itching under the electrodes during the stimulation (44%), headache (close to 25%), and skin redness (almost 18%). Hypomania was observed in two patients (0.5%).
The results suggest that tDCS is a well-suited treatment alternative for MDD, either as a stand-alone treatment or in combination with antidepressant medication.
The complete study results can be found here.
TDCS is the treatment method offered by Sooma for depression (Sooma Depression Therapy, indicated for Major Depressive Disorder) and chronic pain (Sooma Pain Therapy, indicated for Fibromyalgia and chronic neuropathic pain). By using Sooma devices, you ensure that you are performing a safe and effective patient treatment, should it be in the hospital or at patients’ homes, with legal, regulated, tested, and effective equipment that complies with the latest EU regulations and features all the recommended elements listed on the applicable legislation.
Latest news

TGA approves Sooma’s at-home brain stimulation for depression in Australia
Read more
Sooma Medical Announces Pivotal FDA IDE Clinical Trial for At-Home Brain Stimulation Device for Depression Treatment
Read more
Sooma Pioneers the Integration of Brain Stimulation into Primary Care, Improving Access to Early-Stage Depression Treatment
Read more