
Newsroom
The latest news and updates on Sooma tDCS™, neurostimulation treatment, and what’s happening at Sooma Medical.


Sooma Medical Announces Pivotal FDA IDE Clinical Trial for At-Home Brain Stimulation Device for Depression Treatment
Read more
Sooma Pioneers the Integration of Brain Stimulation into Primary Care, Improving Access to Early-Stage Depression Treatment
Read more
Sooma Partners with JSTT to Introduce Brain Stimulation Treatments in Occupational Healthcare
Read more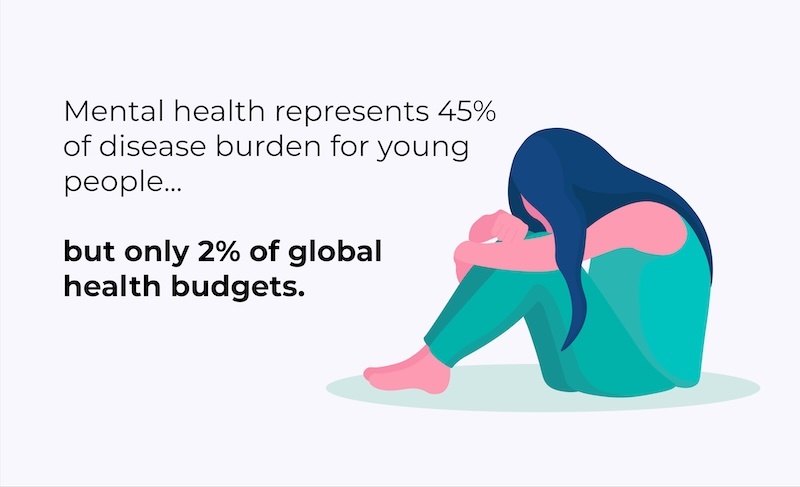
Prioritizing Youth Mental Health: A Call to Action
Read more
Sooma Secures €5M Funding for At-home Brain Stimulation Therapies
Read more
Sooma Secures €5M Funding for At-home Brain Stimulation Therapies
Read more
Dr. Daniel Blumberger Joins Sooma’s Science Advisory Board
Read more
Reflecting on a Year of Innovation and Achievement: Sooma in 2023
Read more
Sooma launches system for remote clinician-monitored brain stimulation
Read more
Sooma becomes the first tDCS device manufacturer to receive European MDR certification
Read more