Reflecting on a Year of Innovation and Achievement: Sooma in 2023

A year of product launches, regulatory victories, and a major milestone in patient treatments.
Join us on a journey through the transformative year that was 2023 as we celebrate a decade of groundbreaking innovation at Sooma. In the ever-evolving landscape of healthcare and technology, our commitment to innovation has been the guiding force propelling us forward. Join us in this retrospective as we revisit the milestones, challenges, and triumphs that defined our 2023.
-
-
-
Sooma Duo Debut
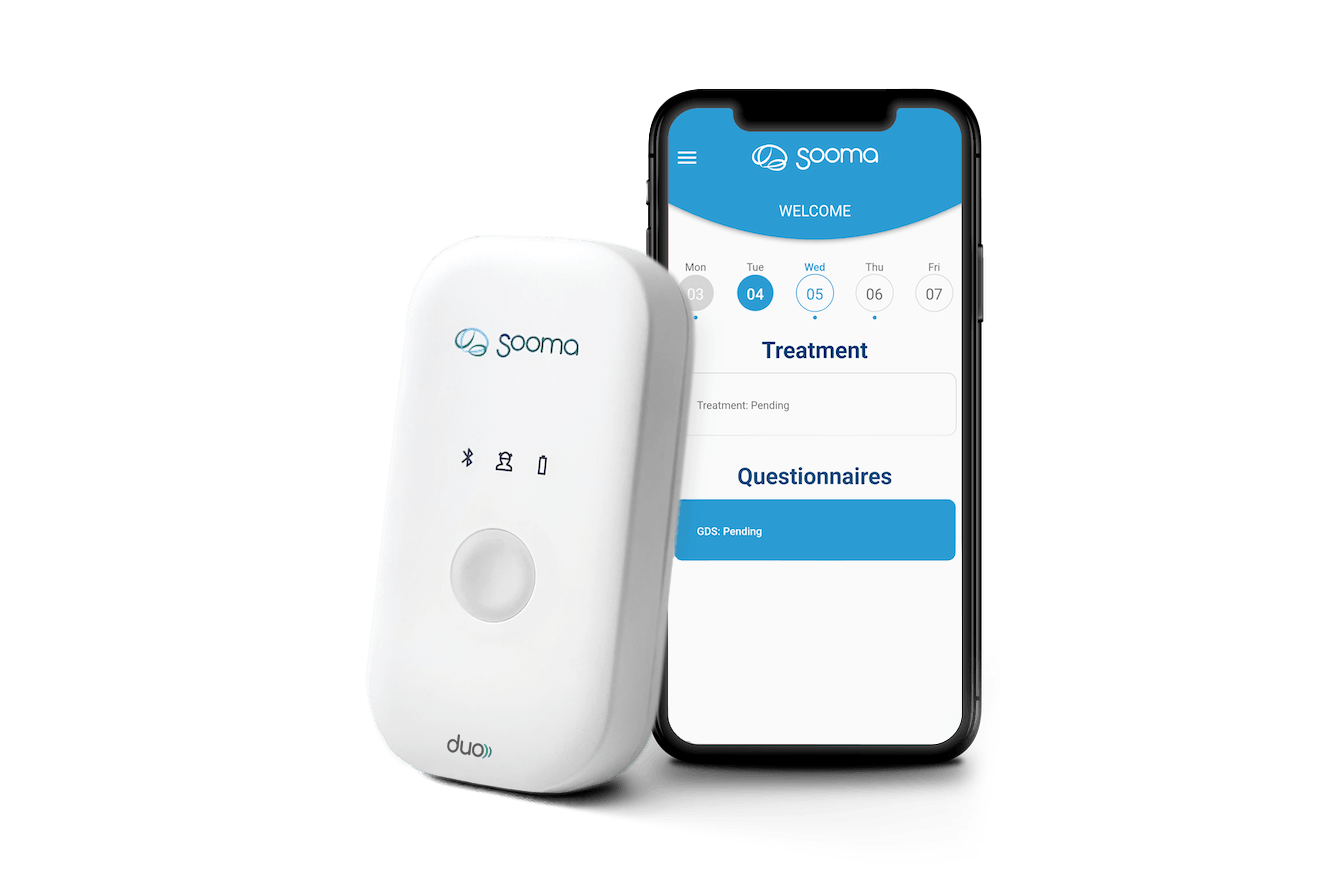
A highlight of 2023 was the unveiling of our groundbreaking product, the Sooma Duo treatment system. This cutting-edge transcranial direct current stimulation (tDCS) device, paired with a state-of-the-art digital platform, marked a new era in patient-centric neuromodulation therapies. Sooma Duo empowers clinicians, engages patients, and ensures treatment compliance, all while meeting the highest quality and safety standards.
Europe’s First Clinical tDCS System to Gain EU MDR Certification

In a groundbreaking achievement, Sooma Duo became Europe’s first and only Clinical tDCS system to carry the MD marking after gaining EU Medical Device Regulation (MDR) certification. This certification not only underscores the safety and efficacy of our product but also positions Sooma as a trailblazer in delivering cutting-edge healthcare solutions.
FDA Breakthrough Device Designation

March 2023 saw a significant milestone as Sooma received FDA Breakthrough Device Designation for our innovative therapies. This recognition highlights the transformative potential of our solutions and paves the way for expedited development and regulatory review, bringing us closer to delivering impactful therapies to those in need.
European Patent Office Recognition
Adding to our list of accomplishments, the European Patent Office (EPO) declared its intention to grant a patent for our groundbreaking electrode placement system. This system, integral to our medical-grade brain stimulation device for depression and chronic pain, stands as a testament to our team’s diligence and solidifies Sooma’s position as a leader in clinical non-invasive neuromodulation therapies.
Spotlight on Team Excellence

Behind every achievement lies the dedication and expertise of our remarkable team. From R&D to clinical process, from quality and regulatory affairs to logistics, each department played a pivotal role in our success. Our team’s resilience and collaborative spirit have been the driving forces behind our innovations.
- In addition to highlighting our team’s professional successes, we are also proud to announce a few smaller additions to the Sooma family, with the births of three happy and healthy babies from Sooma staffers in 2023!
Recognition at home
December saw Sooma gain a significant nod from Mediuutiset, the leading Finnish healthcare news outlet, recognizing Tuomas Neuvonen as one of the Top 100 Influential Leaders in Finnish Healthcare. As one of only ten companies selected for the list, this acknowledgment not only celebrated individual achievements but underscored the collective impact of the entire Sooma team.
Achievement in Patient Care: 20,000+ Prescriptions

-
As the year comes to a close, we are proud to announce our most important achievement. Sooma treatment prescriptions have reached 20,000 cumulative patients. This milestone reflects not just numbers but the lives touched by accessible treatments. By making our therapies available to clinicians and patients globally, we are fostering a new era of inclusive and effective healthcare.
Celebrating a decade of Sooma
As we conclude this re
 trospective journey through 2023, we not only celebrate the achievements of the past year but also mark a decade of Sooma’s relentless pursuit of creating accessible therapies for all. Our 10th anniversary is a poignant reminder of the challenges we’ve overcome, the innovations we’ve introduced, and the lives we’ve touched.
trospective journey through 2023, we not only celebrate the achievements of the past year but also mark a decade of Sooma’s relentless pursuit of creating accessible therapies for all. Our 10th anniversary is a poignant reminder of the challenges we’ve overcome, the innovations we’ve introduced, and the lives we’ve touched.We extend our deepest gratitude to our team, partners, customers, and the clinicians who make it all possible. Looking ahead, we are energized and inspired to continue pushing boundaries, discovering new possibilities, and making a lasting impact on the future of healthcare.
About Sooma:
Established in 2013, Sooma Oy is a Finnish medical device company and the market leader in Transcranial Direct Current Stimulation (tDCS) in clinical use. Sooma therapies are offered by over 150 medical centers across 35 countries, with over 20,000 patients treated.
TDCS is the treatment method offered by Sooma for depression (Sooma Depression Therapy, indicated for Major Depressive Disorder) and chronic pain (Sooma Pain Therapy, indicated for Fibromyalgia and chronic neuropathic pain). By using Sooma devices, you ensure that you are performing a safe and effective patient treatment, should it be in the hospital or at patients’ homes, with legal, regulated, tested, and effective equipment that complies with the latest EU regulations and features all the recommended elements listed on this article.
-
-
Latest news

Sooma Announces Medical Technology Expert Andreas Hartlep as New CEO
Read more
TGA approves Sooma’s at-home brain stimulation for depression in Australia
Read more
Sooma Medical Announces Pivotal FDA IDE Clinical Trial for At-Home Brain Stimulation Device for Depression Treatment
Read more