New U.S. patent granted to Sooma’s electrode structure
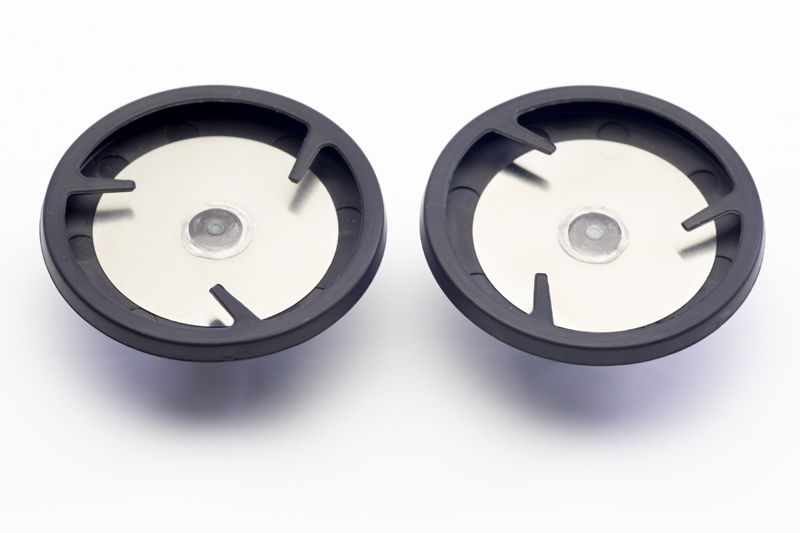
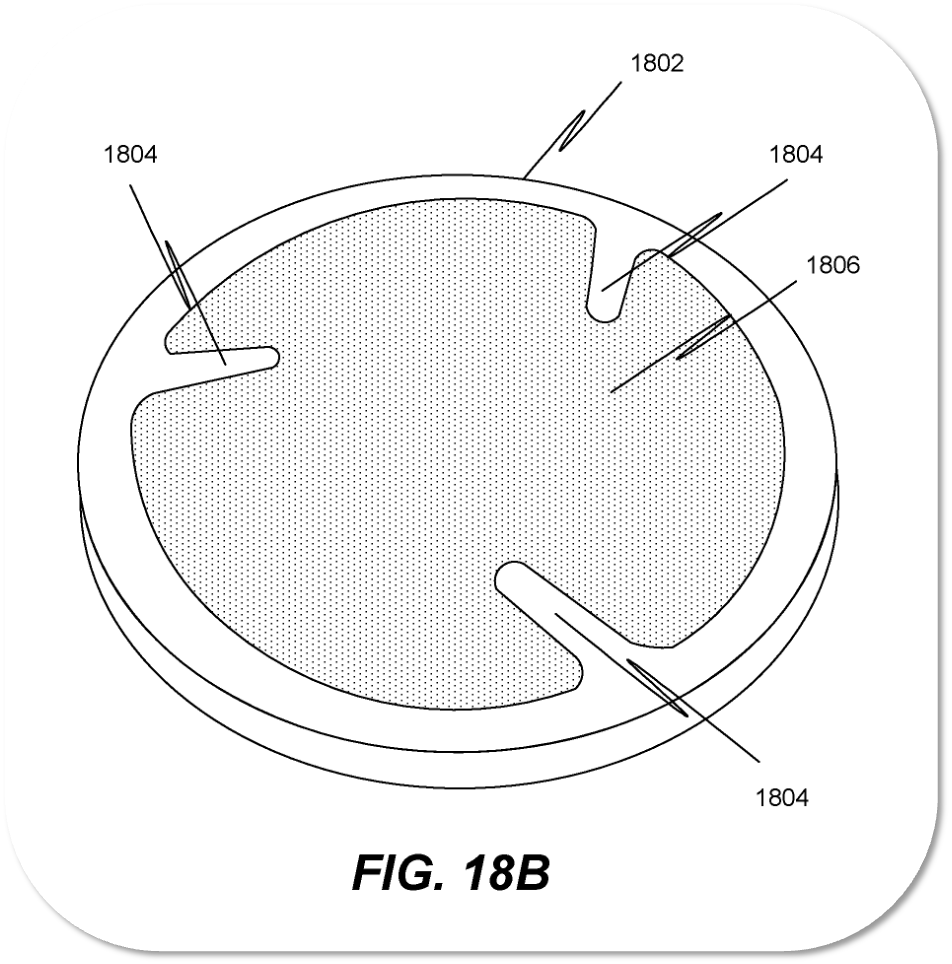
Sooma Medical has been granted a new U.S. patent for its stimulation electrode. The electrode technology is an essential component of neurostimulation devices as it ensures the safety and effectiveness of the treatment sessions.
The electrode technology developed by Sooma, combined with the also U.S. patented hydrogel pads (or ComfoPads), dramatically reduces the resistance between the skin and the electrode, providing a superior accuracy in the delivery of the stimulation dose. Furthermore, the preparation of the treatment session is much easier and faster, and it can be done by the patients themselves at home.
Sooma’s proprietary technologies are the result of years of research with industrial and academic partners, following the strategic goal of focusing innovations on making the patient and clinical use of the devices easier and safer.
With yet another patent secured in the IP portfolio, Sooma Medical strengthens it’s position as the leading provider of home-based neuromodulation solutions with a unique product portfolio. The list of currents patents granted to Sooma come from more than 20 countries around the globe, and future patents are also pending approval.
Sooma tDCS are neuromodulation medical devices that provide a weak electrical current (transcranial direct current stimulation, tDCS) to treat major depressive disorder (MDD), chronic pain and fibromyalgia. The devices are used by professionals and patients in over 100 clinics worldwide.
Sooma products have been approved for medial use in the following markets: EU (Class IIa device), Canada, Mexico, Singapore, Australia, Malaysia, Indonesia and Turkey. In the U.S. the FDA limits the use of the tDCS for research purposes only. Sooma products are distributed in over 30 countries.
Latest news

TGA approves Sooma’s at-home brain stimulation for depression in Australia
Read more
Sooma Medical Announces Pivotal FDA IDE Clinical Trial for At-Home Brain Stimulation Device for Depression Treatment
Read more
Sooma Pioneers the Integration of Brain Stimulation into Primary Care, Improving Access to Early-Stage Depression Treatment
Read more