Sooma Receives Intention to Grant Patent from EPO for Its Innovative Electrode Placement System, Reinforcing Patent Portfolio
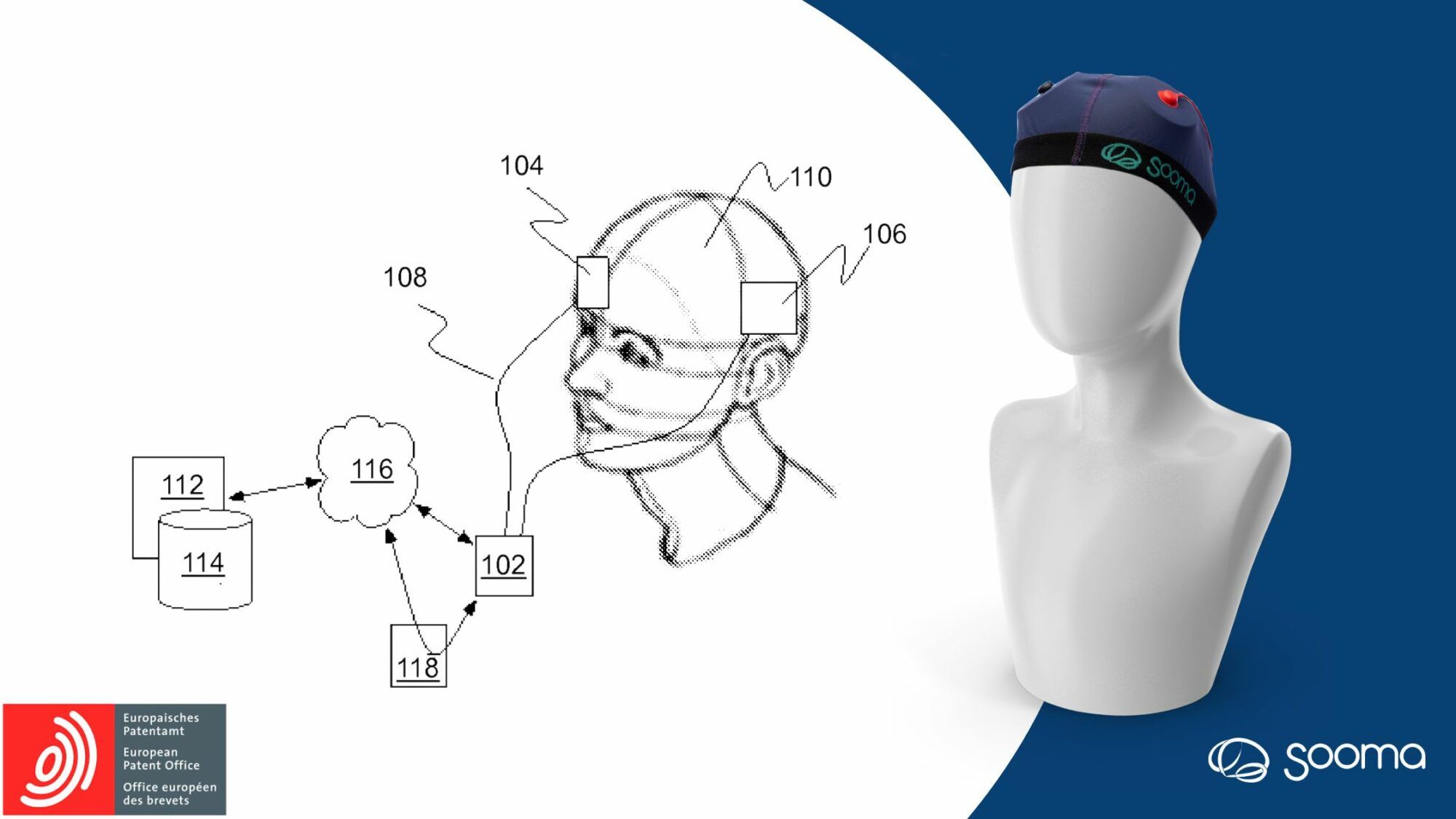
The EPO patent intention serves as a testament to Sooma’s dedication to advancing the efficacy and precision of tDCS therapy.
At Sooma Medical, we are delighted to announce that the European Patent Office (EPO) has declared its intention to grant a patent for our groundbreaking electrode placement system used by our medical-grade brain stimulation device for depression and chronic pain. This significant development is a recognition of our team’s diligent work and further solidifies Sooma’s position as a leader in the field of clinical non-invasive neuromodulation therapies for psychiatric and neurological disorders.
The EPO’s intention to grant patent validates Sooma’s dedication to improving patient care by providing accessible brain stimulation therapies with a developmental focus on treatment efficacy and precision. This achievement adds to the company’s already extensive patent portfolio in the field of neurostimulation and empowers medical professionals to optimize treatment outcomes and improve patient care.
“Our patents are focused on ensuring the safety and effectiveness of our therapies,” says Tuomas Neuvonen, Sooma CEO and co-founder. “This recognition supports our ongoing efforts towards ensuring the precise reproducibility of the intended stimulation dose, regardless of whether the patient is being treated in-clinic or is self-administering treatment at home, therefore improving the safety and efficacy of the therapy.”
This validation, together with the recently obtained Breakthrough Device designation from the FDA in the United States, strengthens our position as the preferred choice of healthcare professionals around the globe for clinical-grade tDCS devices.
We invite medical professionals to learn more about our innovative tDCS therapies by visiting our website at soomamedical.com or our experts at info@soomamedical.com.
About Sooma:
Established in 2013, Sooma Oy is a Finnish medical device company and the market leader in Transcranial Direct Current Stimulation (tDCS) in clinical use. Sooma therapies are offered by over 150 medical centres across 35 countries, with over 15,000 patients treated.
TDCS is the treatment method offered by Sooma for depression (Sooma Depression Therapy, indicated for Major Depressive Disorder) and chronic pain (Sooma Pain Therapy, indicated for Fibromyalgia and chronic neuropathic pain). By using Sooma devices, you ensure that you are performing a safe and effective patient treatment, should it be in the hospital or at patients’ homes, with legal, regulated, tested, and effective equipment that complies with the latest EU regulations and features all the recommended elements listed on this article.
Latest news

TGA approves Sooma’s at-home brain stimulation for depression in Australia
Read more
Sooma Medical Announces Pivotal FDA IDE Clinical Trial for At-Home Brain Stimulation Device for Depression Treatment
Read more
Sooma Pioneers the Integration of Brain Stimulation into Primary Care, Improving Access to Early-Stage Depression Treatment
Read more