Sooma tDCS - Complete therapy solution
See how it works
Designed to treat both in clinics and at home.
A reliable therapy solution
Sooma tDCS offers a complete therapy solution indicated for the treatment of Major Depressive Disorder, Chronic Neuropathic pain, and Fibromyalgia. The solution features a high-quality stimulation device and accessories that ensure dose precision and replicability. Sooma tDCS enables home-based stimulation and faster preparation, allowing savings in costs and time.
Read more how Sooma tDCS was successfully used in the first published placebo-controlled at-home tDCS study (Hyvärinen et al, 2016).
Contact us for detailed technical specifications.
Developed by professionals, for professionals
Sooma tDCS has been developed in co-operation with healthcare professionals to meet clinical routine use. The solution follows three key principles:
- The stimulation dose must be controlled
- The stimulation target must be accurate
- The patient must be able to self-administer the stimulation
Sooma tDCS has been approved for both clinic and at-home use. To date, over 100 000 stimulation sessions have been given using Sooma tDCS for treatment and research purposes.



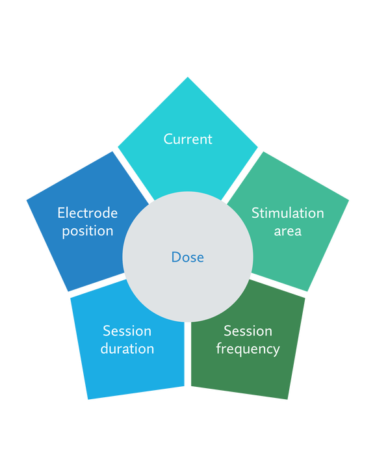
Stimulation dose control
The effects of tDCS are based on transcranial stimulation, which is an electrical current to the patient’s brain. As the current needs to pass through layers such as hair and skin that form resistance, the stimulator needs sufficient power to deliver the required current. Sooma tDCS is equipped with automated resistance monitoring that keeps the current delivery constant. The session dose in tDCS is not just the electrical output though but also involves where and for how long the stimulation is delivered, where we come to the question of accuracy.
By using user-replaceable batteries as a power source, we can extend the lifetime of the device beyond the lifetime of a chargeable battery.
Accuracy of the stimulation target
Placing of the electrodes in the right place is highly important for the stimulation. tDCS is a diffuse stimulation method, meaning that the current will spread to large areas in the brain under the electrode. When the traditional way is to measure the electrode locations which requires a technician or a trained nurse and takes a long time, Sooma head caps with pre-configured placements allows a reliable electrode placement precsisly and effortless. This works for every session, also when treatment preparation is done at home without assistance.
Also, the size of the used electrodes is crucial to make the stimulation area precise. Sooma Comfort stimulation accessories are designed to limit electrolyte spread and keep the stimulated area under the electrodes. This ensures that the dose is delivered to the targeted brain area for the best therapeutic effect.

Self-administration at home
Sooma tDCS has been designed for home usage and it tackles the complexity that may occur when moving from clinic to remote use. Because the environment can be complex, our approach is to make the stimulation system simple. The solution includes several automated safeguards to ensure its correct use, and it is highly shielded from any electromagnetic disturbance that would affect its function. Also, the solution knows how many sessions the patient has done and ensures that the patient uses only the protocol specified by the responsible clinician.
After being trained by a nurse during the first treatment session, the patient can orchestrate the at-home treatment. The training can also be done via video connection. When a home treatment course is ongoing, the patients’ adherence and progress can be followed remotely. This provides a clinician with detailed information on the patient’s condition and the ability to adjust the treatment based on day-to-day progress.

Enquiry form
Please note that Sooma tDCS is sold only to medical professionals. If you are a patient wishing to receive tDCS treatment, please inquire your physician about the possibility or fill out our contact form and we can help you find a provider.